25 Years of BDD: From Spin-out to Lean Clinical Development™

On 15 November 2025, BDD Pharma Ltd celebrates 25 years since incorporation; a journey that started as a spin out from Strathclyde University and has grown into a world-class drug delivery company, combining formulation, GMP manufacture and Phase I clinical trials under one roof.
What began as a bold idea to see how medicines really behave in the human body has become a fully integrated Lean Clinical Development™ pathway that helps partners around the world turn complex drug delivery challenges into real clinical answers.
How it all began:
Twenty-five years ago, Professors Howard Stevens and Clive Wilson stood in the basement of Glasgow Royal Infirmary, watching their new clinical research centre take shape. Just a year earlier, Howard had secured a research grant to convert the unused space into a dedicated facility where, instead of relying solely on in vitro experiments, their team could for the first time run trials of novel drug formulations in human subjects.
That vision – to link pharmaceutical science and clinical practice in one place – became the foundation for everything that followed. Their PhD students and research fellows now had access to a centre where they could test ideas in man, not just in the lab, and directly observe how dosage forms behaved in real patients, in real time.
Founder’s reflection
“Twenty-five years ago, Clive Wilson and I marvelled at the sight of our new clinical research centre taking shape in a basement at Glasgow Royal Infirmary. For the first time, we could move from in-vitro experiments to testing novel formulations in human subjects – directly linking pharmaceutical and clinical sciences in our own centre.”
— Professor Howard Stevens, Founder and Director, BDD Pharma Ltd.
From Bio-Images to BDD Pharma Ltd.
![]()
The company was originally incorporated as Bio-Images Research Ltd on 15 November 2000, reflecting its roots in clinical imaging and early-phase research.
From the beginning, the team’s work was heavily focused on gamma scintigraphy – a powerful imaging technique used to track how radiolabelled formulations move, disintegrate and release drug in the body. This capability quickly attracted collaborations with industrial partners and clinicians across a wide range of therapeutic areas.
Founder’s reflection
“Those early years were defined by gamma scintigraphy. Our clinical centre became a hub where industrial drug delivery scientists and clinicians could see their formulations in action – and often be surprised by what the images revealed.”
— Professor Howard Stevens
 As demand grew, so did the vision. A wealth of knowledge was being built up with the testing of so many varied formulations in humans, knowledge that translated to a solid understanding of what products would work in humans and what was required to be confident of clinical success.
As demand grew, so did the vision. A wealth of knowledge was being built up with the testing of so many varied formulations in humans, knowledge that translated to a solid understanding of what products would work in humans and what was required to be confident of clinical success.
That knowledge was harnessed in the development of a wholly unique timed drug delivery platform technology that we now call OralogiK™. A technology that was designed to deliver drugs at a specific time post dosing and completely independent of biological variables such as pH, agitation and fed/fasted states.
This technology was the foundation of our sister company Drug Delivery International (DDI) Ltd in 2010 to out-licence this novel patented controlled-release technology, and to offer formulation development services (coming from the aforementioned knowledge) alongside clinical imaging.
By 2017, the two companies were formally brought together when Bio-Images Research and DDI and combined as BDD Pharma Ltd, creating a single organisation that could offer clients both drug delivery expertise and clinical capabilities in one place.
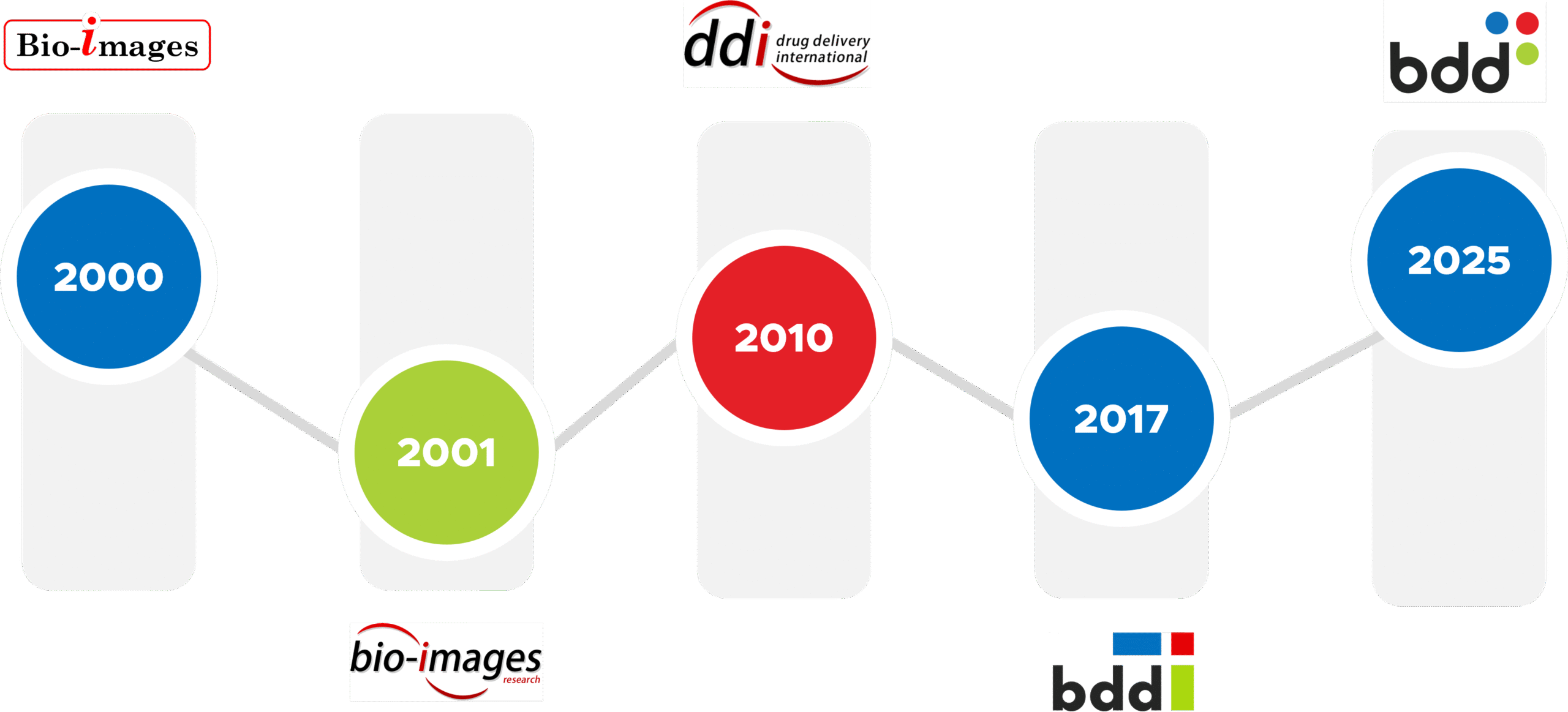
2000 – Bio-Images Research Ltd incorporated
2001 – Clinical research centre at GRI opens
2010 – Drug Delivery International (DDI) founded
2017 – DDI and Bio-Images merge to form BDD Pharma Ltd.
2025 – New GMP facility opens, and Lean Clinical Development™ offering expands
Science that shaped us: seeing medicines in motion
At the heart of BDD’s story is a simple but powerful idea: understanding what happens in the body is crucial – what you see in vitro doesn’t always match what happens in vivo.
Gamma scintigraphy became the company’s defining tool for answering that question. By radiolabelling dosage forms and capturing images as they travel through the body, BDD’s teams could see exactly where and when a formulation disintegrated, released drug, or failed to behave as expected.
Over more than two decades, that work has spanned:
- Immediate and modified-release tablets and capsules
- Complex oral formulations such as multi-layer and tablet-in-tablet systems
- Respiratory and pulmonary products
- Liquids, suspensions, eye drops and nasal products
Each study added another layer of real-world evidence – challenging assumptions about pH-dependent coatings, gastric emptying, capsule performance, and more.
Founder’s reflection
“Our 25-year journey has always been underpinned by the integration of top-quality science with real commercial need. That was true on the day we took our first steps at Glasgow Royal Infirmary, and it remains true at BDD today.”
— Professor Howard Stevens
From imaging specialists to Lean Clinical Development™
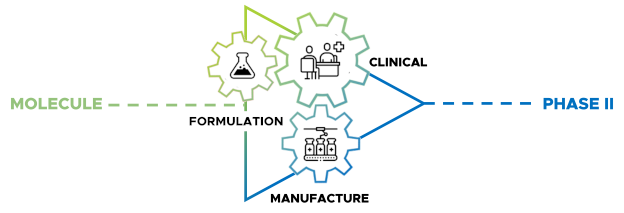
As BDD’s experience grew, one message kept coming back from clients: they didn’t just need data from a single study – they needed a joined-up pathway from formulation through to clinical proof-of-concept.
In response, BDD gradually built out an integrated model that combines:
- Formulation and biopharmaceutics expertise – specialising in complex and controlled-release oral dosage forms
- Phase-appropriate GMP manufacture – small-scale, flexible production of IMP and matching placebo for early-stage trials
- Phase I clinical trials and gamma scintigraphy – including first-in-human, PK, food-effect and adaptive designs, all run at BDD’s own clinical site
These components were brought together under a single, modular pathway: Lean Clinical Development™. The aim is simple – to cut unnecessary handovers, remove duplicated work, enable fast adaptations to formulations in response to clinical read outs and deliver faster, smarter early clinical data for our partners.
Recent investments have strengthened this further. In 2025, BDD opened a brand-new 122 m² GMP manufacturing facility, supported by a £2m funding package, expanding capacity for small-batch clinical supply and underpinning the Lean Clinical Development™ approach.

CEO Carol
I remember well meeting with Howard and the Bio-Images team in Ad Lib, a popular restaurant near to the University. At that time, I was a commercialisation officer at Strathclyde University tasked with spinning out a company to bring OralogiK to the market. Bio-Images had a small lab in their clinical facility, and we debated if we could use that as a starting ground for DDi – we did. Within months, we had two contracts for the development of complex formulations which has always been our speciality, and we continued to develop OralogiK. When I think of the products that we were able to develop for the market in our tiny back lab I smile, our science, our problem-solving minds and our tenacity has always been our strength, that’s why we still confidently say that we can Deliver the Impossible. Now with our purpose-built QC and development labs collocated with our brand-new scale up GMP facility, I am proud to be able to support our partners in bringing their innovative product ideas to life for patients across the globe”
– Dr Carol Thomson, CEO of BDD and co-founder of DDi,
Novel Oral Tablet Technologies
Alongside its service model, BDD has developed and refined proprietary oral drug delivery platforms that embody the same principle: using our unique knowledge of the GI tract to inform better, more predictable release profiles.
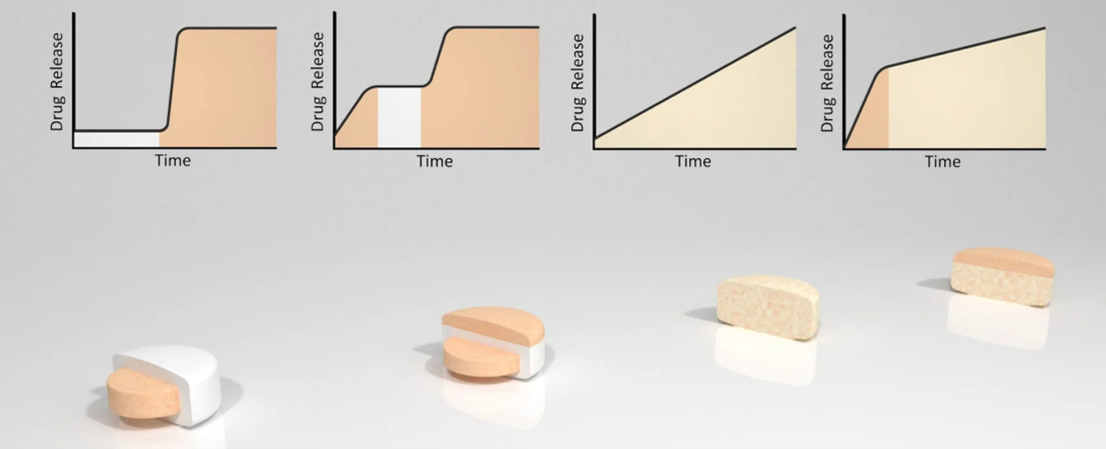
is BDD’s patented, erosion-based tablet-in-tablet technology. By compressing an erodible barrier layer around a drug-containing core, it enables:
- Precise, time-controlled drug release
- Pulsatile or multi-pulse profiles
- Complex bi- or tri-phasic delivery of one or more actives
- Performance independent of GI pH or hydrodynamics
This platform has formed the basis of numerous collaborations with partners across the globe. One standout milestone in the anniversary year is the progress of CTx-1301, Cingulate’s once-daily, triple-pulse ADHD therapy built on BDD’s OralogiK™ technology. In 2025, the US FDA accepted CTx-1301’s New Drug Application for review, with a PDUFA date set for May 2026 – a major step toward bringing a new generation of ADHD treatment to patients.
Founder’s reflection
“The early patents from our time-delayed oral delivery work at Strathclyde formed the basis for OralogiK™. Seeing that science now helping partners like Cingulate bring once-daily, triple-pulse treatments towards market is a hugely proud moment for all of us.”
— Professor Howard Stevens
Real World Impact
Over 25 years, the BDD team has:
- Conducted hundreds of early-phase clinical and scintigraphic studies across oral and respiratory routes
- Developed and protected global patent families for OralogiK™ and CologiK™, with coverage in major markets worldwide
- Built a reputation as a specialist partner for complex oral drug delivery, backed by in-house clinical trial capabilities and adaptive study designs
You’ll also find BDD technologies embedded in programmes that span CNS, metabolic disease, GI, inflammatory conditions and more – always with the same goal: better real-world performance, informed by early stage clinical data.
The people behind BDD
Behind the milestones, patents and publications is a team of scientists, clinicians, GMP specialists, project managers and support staff who have shaped BDD’s identity over the past 25 years.
From the very first PhD students working late in the Glasgow Royal Infirmary basement, through to the formulation, GMP and clinical teams at BioCity and Glasgow today, BDD has always been led by science, and driven by people who care deeply about getting better medicines to patients.
Many of those who were there in the early days are still part of the organisation. New colleagues have joined from industry and academia, bringing fresh perspectives while keeping the same values: curiosity, rigour, collaboration and a practical focus on solving real problems for partners.

Looking ahead: the next 25 years
If the first 25 years were about proving what’s possible when you combine imaging, formulation and clinical trials, the next 25 will be about scaling that model and applying it to ever more complex challenges.
Looking forward, BDD is focused on:
- Expanding its Lean Clinical Development™ pathway to support increasingly complex molecules and regimens
- Further enhancing its imaging and clinical data readouts
- Growing GMP capacity and flexibility for small, smart batches that support adaptive trial designs
- Continuing to evolve OralogiK™ and Cologik™ and developing new formulation solutions to longstanding dosing problems.
CEO Carol
“This is such an exciting time for BDD with our first OralogiK product coming close to market launch and many others following in its path, enabling value add medicines to make a real difference to patient lives. We have not lost our love for science or our passion for developing enabling drug delivery systems which we continue to do. We have built a first class team who are committed to speeding up the development of new medicines using our Lean Clinical Development pathway and I genuinely do not think that there is anywhere else in the world where developers analysts and clinical teams work together so collaboratively to get to the right product faster. I am looking forward to continuing to grow BDD and to help bring more great products to the market with our characteristic enthusiasm, scientific fervour and readiness for a challenge.”
– Dr Carol Thomson
As we mark this 25-year milestone, one thing hasn’t changed since that first day: BDD still exists to bring top-quality science and real commercial need together, so that more patients can benefit from better medicines, sooner.