BDD are market leaders in gamma scintigraphy with a proven track record and a global client base.
For more than 20 years, BDD have helped our clients with exciting and challenging studies to understand better how their products perform in man. We have performed studies across a wide range of dosage forms including tablets, capsules, respiratory products, drinks, eye drops and nasal administrations.
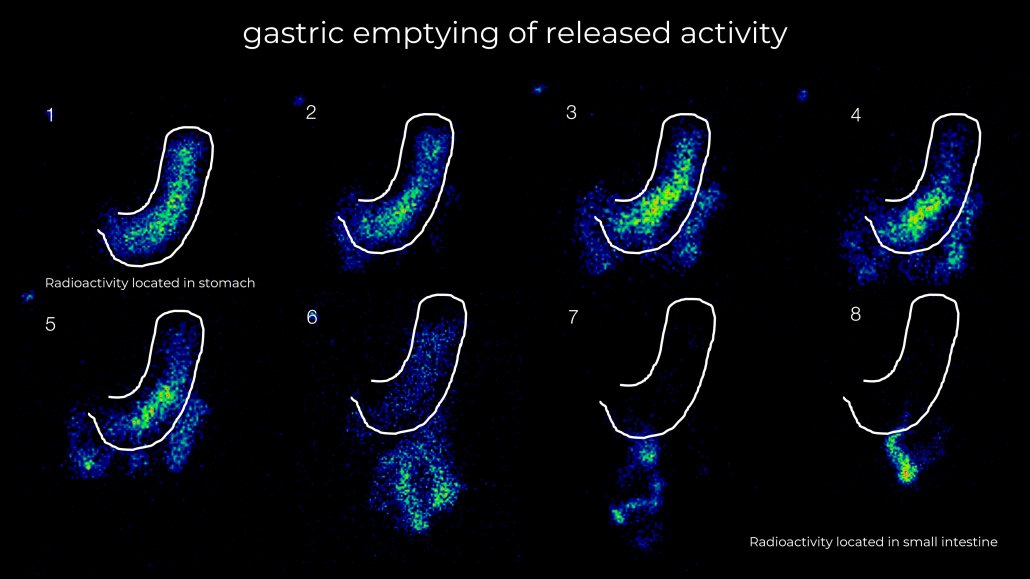
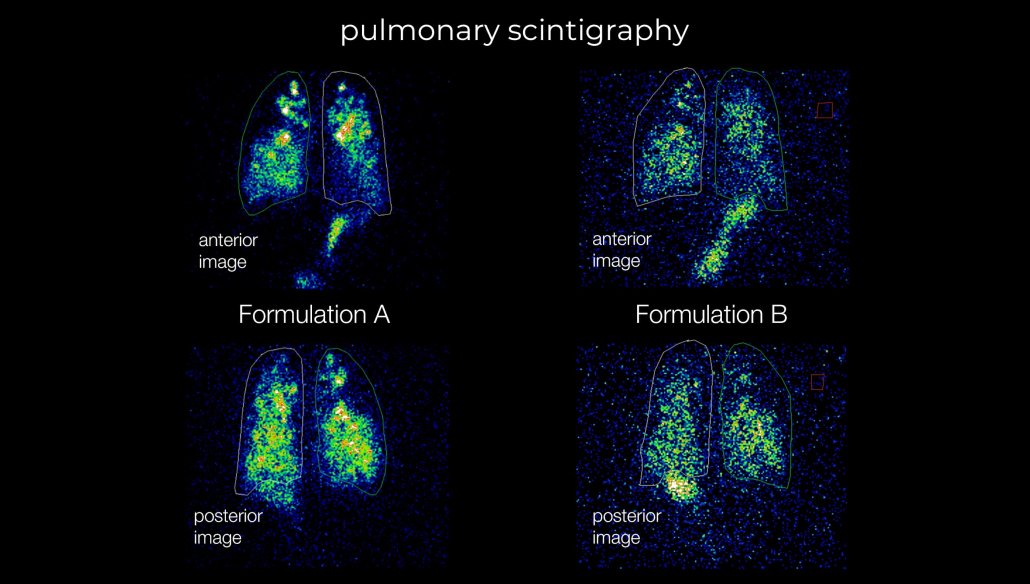
Our studies open up a range of in-vivo data that is critical to product performance that cannot be obtained in vitro or through simulation studies. Data such as site and rate of release, gastric emptying, residence time and even rate of swallowing alongside pharmacokinetic profiles can offer unparalleled insight into your product behaviour.
Our expert staff have years of experience in analysing and interpreting scintigraphic imaging data to work with you to understand fully the data obtained from these studies.
Gamma Scintigraphy can help you to:
- Understand and visualise the performance of your dosage form in man
- Rapidly screen formulations and provide data–backed decision making during early-stage formulation development
- Evidence targeted drug release
- Provide data for future regulatory submissions